Research Introduction
Our lab has revealed the molecular mechanism of macropinocytosis and the physiological relevance in the past 5 years (see our publications). While continuing the cell biology research as our main direction, we are trying to apply our findings to other fields, such as immunology, cancer biology, and nephrology.
Macropinocytosis is large-scale endocytosis, by which cells can ingest or “drink” extracellular nutrients efficiently. We have shown that growth factor stimulation induces macropinocytosis and revealed the molecular mechanism (JCS 2009, Frontiers 2015). Growth factor receptor signaling and the overall metabolic status of the cell are coordinated in the cytoplasm by the mechanistic target-of-rapamycin complex-1 (mTORC1), which positively regulates protein synthesis and negatively regulates molecular salvage pathways such as autophagy.
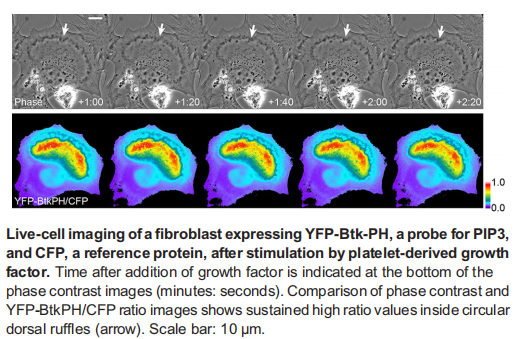
Our studies have shown that growth factor-stimulated macropinocytosis is essential for PI3K/mTORC1 pathway (JCB 2015, JLB 2017, JCS 2018, CMLS 2018). Based on these achievements, we are now interested in the roles of macropinocytosis in macrophage biology (Projects 1 and 2), cancer development (Project 3), and podocyte functions (Project 4).
Project 1 Study of the molecular mechanism of “Suqidlysis”
Lysosomes are dynamic organelle and form tubule-like structures (tubular lysosomes: TLs) in macrophages. We have observed that macropinosomes are wrapped by TLs in growth factor-stimulated macrophages. Because of the unique shape we name the fusion process “Squidlysis”. We are currently investigating the molecular mechanism of TL dynamics using the Squidlysis process as the subject.
Reference:
Yoshida S, Pacitto R, Yao Y, Inoki K and Swanson JA. (2015). Growth Factor Signaling to mTORC1 by Amino Acid-laden Macropinosomes. Journal of Cell Biology. 211: 159-172. doi: 10.1083/jcb.201504097
Project 2 Dissect the role of macropinocytosis in cytokine synthesis
Recent researches including ours (US Patent Application 2008) show that the PI3K/mTORC1 pathway regulates cytokine expression in phagocytes. Given that our finding shows that macropinocytosis regulates PI3K/mTORC1 pathway and that LPS induces macropinocytosis in macrophage, we hypothesize that macropinocytosis modulates cytokine expression via the PI3K/mTORC1 pathway.
Reference:
[1] Pacitto R, Gaeta I, Swanson JA and Yoshida S*. (2017). CXCL12-induced macropinocytosis modulates two distinct pathways to activate mTORC1 in macrophages.Journal of Leukocyte Biology. 101: 683-692 doi: 10.1189/jlb.2A0316-141RR
[2]Yoshida S, Pacitto R, Yao Y, Inoki K and Swanson JA. (2015). Growth Factor Signaling to mTORC1 by Amino Acid-laden Macropinosomes. Journal of Cell Biology. 211: 159-172. doi: 10.1083/jcb.201504097
[3]Koyasu S, Ohtani M, Sasakawa C, Yoshida S (2008).Inhibitor of PI3 kinase-dependent inflammatory cytokine synthesis and method for inhibiting the same. US Patent Application. Number: 20080317875.
Project 3 Determine the role of macropinocytosis in cancer development
Our current (JCS 2018) and preliminary research show that the EGF pathway is regulated by macropinocytosis in the breast cancer cell. By the combination of biochemistry and imaging, we investigate the pathological roles of macropinocytosis in cancer development.
Reference:
Yoshida S*, Pacitto R, Sesi C, Kotula L, and Swanson J*. (2018). Dorsal Ruffles Enhance Activation of Akt by Growth Factors.Journal of Cell Science. 131: jcs220517 doi: 10.1242/jcs.220517
Project 4 Identify the role of macropinocytosis in podocyte
We are interested in the cell biology of podocytes. Podocytes are highly specialized epithelial cells, which cover the glomerulus as a part of the filter function of the kidney. It has been shown that podocytes induce macropinocytosis, but the molecular mechanism and the physiological functions are unknown.
Reference:
[1] Yao Y, Wang J, Yoshida S, Nada S, Okada M and Inoki K. (2016). Role of Ragulator in the Regulation of Mechanistic Target of Rapamycin Signaling in Podocytes and Glomerular Function. Journal of American Society of Nephrology. 27: 3653-3665 doi: 10.1681/ASN2015010032
[2]Huber TB, Edelstein CL, Hartleben B, Inoki K, Jiang M, Koya D, Kume S, Lieberthal W, Pallet N, Quiroga A, Ravichandran K, Susztak K, Yoshida S and Dong Z. (2012). Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 8: 1009-1031.
[3]Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. (2011). mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. Journal of Clinical Investigation.121:2181-2196.